In What Circumstances Is It Necessary To Filter A Hot Recrystallization Solution?
1.5E: Hot Filtration
- Page ID
- 93377
Hot Filtration Overview
A hot filtration is generally used in some crystallization, when a solid contains impurities that are insoluble in the crystallization solvent. It is also necessary in crystallization when charcoal is used to remove highly colored impurities from a solid, equally charcoal is so fine that it cannot be removed past decanting.
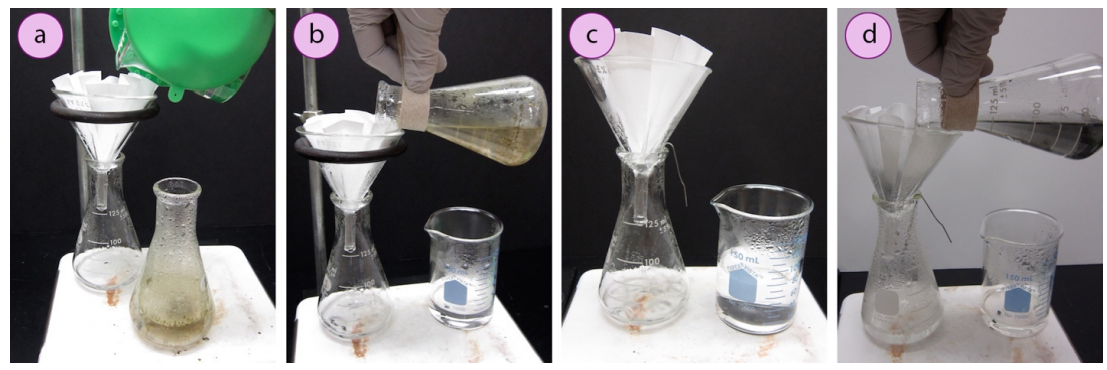
A hot filtration is performed by first pouring a few \(\text{mL}\) of solvent through a funnel containing a "fluted filter paper". A fluted filter newspaper has many indentations and loftier area, which allows for a fast filtration. The funnel is allowed to get hot, while the mixture to be filtered is brought to a eddy. The boiling mixture is so poured through the filter paper in portions (Figures 1.81b+d).
.png?revision=1&size=bestfit&width=1107&height=370)
Information technology is best to use a ring clench to secure the filtration funnel, although the funnel could besides be simply placed atop the flask. If not using a ring clamp, it is recommended to place a bent paper clip betwixt the flask and funnel to let for displaced air to escape the bottom flask as liquid drains (Figure ane.81c+d). Without a band clamp, the setup is more than prone to tipping and and then using a band clamp is considerably safer.
A hot filtration is used for filtering solutions that volition crystallize when allowed to cool. It is therefore important that the funnel is kept hot during filtration through contact with hot solvent vapors, or crystals may prematurely form on the filter paper or in the stem of the funnel (Figure 1.82).
.png?revision=1&size=bestfit&width=1103&height=369)
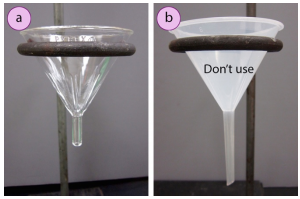
Crystallization on the filter paper can clog the setup and cause a loss of yield (as the filter newspaper will be afterward thrown away). Crystallization in the stem hinders filtration, and can act as a plug on the bottom of the funnel. An reward of hot filtration is that the boiling solvent in the filter flask helps to dissolve crystals that prematurely form in the stem of the funnel. With hot filtration, it is advised to apply a short-stemmed funnel (Figure 1.83a) or stemless funnel if available, instead of a long-stemmed funnel (Figure one.83b), as material is less likely to crystallize in a short or absent stalk.
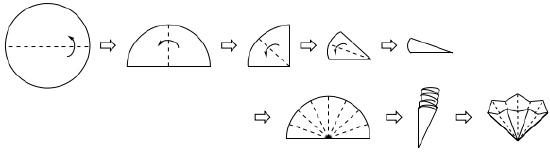
Equally it is essential that a solution filters speedily before it has a chance to cool off in the funnel, a "fluted filter paper" (Effigy 1.84b+c) is usually used instead of the quadrant-folded filter newspaper sometimes used with gravity filtration (Figure 1.84a). The greater number of bends on the fluted filter paper translate into increased surface expanse and quicker filtration. The folds also create space between the filter paper and glass funnel, assuasive for displaced air to more easily leave the flask as liquid drains.
.png?revision=1&size=bestfit&width=1106&height=373)
Step-by-Step Procedures
Hot filtration is oft used with crystallization, and this process should be inserted subsequently the dissolution step, just before setting aside the solution to slowly cool.
.png?revision=1&size=bestfit&width=1104&height=369)
Ready the Filtration Setup
- Obtain a stemless or short-stemmed funnel (Figure i.85a), and insert information technology into a ring clamp, attached to a band stand or latticework (or alternatively, obtain a bent paper prune for the purpose shown in Effigy 1.85b).
- Flute a filter newspaper of the correct size for your funnel into an accordion shape (instructions are in Effigy ane.86 and the resulting piano accordion is in Effigy i.85c). When placed in the funnel, the newspaper should not be shorter than the elevation of the funnel, or the solution might slip past the filter paper when poured.
- With a clean Erlenmeyer flask of the correct size for the crystallization below the funnel and on the heat source, pour a few \(\text{mL}\) of hot solvent into the funnel (Figure i.85d).
- If using a ring clench, adjust the clamp so that there is a pocket-size gap betwixt the oral fissure of the Erlenmeyer and bottom of the funnel: this allows for air to exist displaced when liquid flows into the flask. If the gap is too big, hot vapors will escape without heating the funnel.
- If not using a band clamp, place a bent paper prune between the flask and funnel (Figure one.85b).
- Allow the solvent to boil and get the entire setup hot. If using charcoal, insert that procedure now.
.png?revision=1&size=bestfit&width=903&height=302)
Filter the Solution in Portions
- When the filter flask is quite hot, and the solution to be filtered is boiling, cascade the humid mixture into the filter funnel in portions. Touch the flask to the filter paper in the funnel as you pour (Figure ane.87a).
- Safety annotation: the flask may be quite hot, and hot vapors may scald your hand as you lot pour (pour sideways and so your paw is not to a higher place the funnel). If the flask is too hot to agree with your hands, use a "newspaper towel holder" to hold the flask (Effigy 1.87b):
- Fold a section of paper towel over several times such that the resulting strip is roughly one inch wide. If desired, secure the strip together using a few pieces of tape.
- When holding a flask, the paper towel holder should be beneath the lip of the flask. In this way, liquid will not wick toward the paper towel when pouring (towel remains dry out in Effigy i.87a), but moisture with the besides broad towel in Figure 1.87c).
.png?revision=1&size=bestfit&width=943&height=317)
- When non pouring the mixture to exist filtered, return the flask to the heat source (Figure 1.88a).
- When the mixture is completely filtered, ready the empty flask on the benchtop ( safe note: do not heat an empty flask, or it may crack). Inspect the funnel: if crystals are seen on the filter paper (as in Figure i.88b), rinse with a few \(\text{mL}\) of boiling solvent to dissolve them. A rinse is non needed in Effigy 1.88c.
- Inspect the filtrate (the liquid that has gone through the filter paper). If charcoal was used and the filtrate is grey, or you can meet fine blackness particles, then charcoal passed through the filter paper either through a hole or past using the wrong filter mesh size. If classmates do non have greyness in their solutions, information technology was likely a pigsty. Echo the hot filtration step with a new filter paper and flask.
Hot Filtration Summary
Tabular array 1.eleven: Procedural summary for hot filtration.
In What Circumstances Is It Necessary To Filter A Hot Recrystallization Solution?,
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_Lab_Techniques_(Nichols)/01%3A_General_Techniques/1.05%3A_Filtering_Methods/1.5E%3A_Hot_Filtration
Posted by: tharpsandint.blogspot.com


0 Response to "In What Circumstances Is It Necessary To Filter A Hot Recrystallization Solution?"
Post a Comment